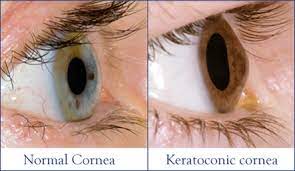
Clinicians have long known the connection between genetics and eye care. We have routinely performed a “genetic survey” in every patient we have ever seen. Yes, we routinely ask our patients with questions as, “Do you have a family history of glaucoma?” We have recognized the importance of genetic information all along, although we usually call it as family history.
As genetic testing knowledge has advanced in the last years, we are now accomplished of questioning a patient about their known family history, but then confirmed that information objectively by looking at genetic markers that predispose patients to certain conditions. This new capability opens the doors to new methods to preventative medicine in the speciality contact lens practices.
Genetic Testing for the Eye
There have been developments in genetic markers for eye disease for at least the last 40 years. In the early days, a noteworthy focus was on gene expression and the potential influence on cells, and most of the breakthroughs focused on the most infrequent eye conditions. For example, in 1993 there was enthusiasm around a team locating the mutation linked to Stargardt’s disease, and by 1998 there were approximately $70 million in grants to the National Eye Institute (NEI) for genetic studies into eye diseases.1,2
The identification of rare diseases that have particular loci was able to advance faster than other conditions. Retinal disorders with targeted loci and their high level of associated vision loss specifically have received a noteworthy volume of investment and consideration; most notably, retinitis pigmentosa (RP) and some syndromes from mutations in the RPE65 gene that most often afflict children.
The progresses in testing, targeting, and therapy resulted in the FDA approval of voretigene neparvovecrzyl (Luxturna, Spark Therapeutics www.sparktx.com) in 2017. The first gene therapy of its type permitted anyplace in the world for confirmed biallelic RPE65 mutation-associated retinal dystrophy. While the prevalence of this genetic disease is significantly less than most other eye diseases, the potential that this process of testing and therapy development gives is substantial. There are several labs today that offer testing for published eye-related genes, including Arctic Medical Laboratories, which provides two types of tests for macular degeneration risk.
Genetic Testing for the Cornea
The first commercially available test for corneal dystrophies was completed by Avellino Labs (www.avellino.com) in 2008. This first test only tested for one TGFBI mutation known to cause Avellino dystrophy (now also known as GCD2). The “Avellino Test” was modified to embrace five mutations of the TGFBI gene.
Initial scepticism over genetic testing for corneal dystrophies subsequently turned into belief, as more significant positive outcomes were being found with increased testing. To date, over 770,000 tests have been done, with over 1,100 positively identified patients. The rate of corneal dystrophies does not make them common diseases, but the prevalence is significantly superior than the 1:2,000 prevalence ratio that defines a condition as rare, by European standards.
In 2019, Avellino Precision Medicine launched the AvaGen test. This test looks for further than 70 mutations of the TGFBI gene for corneal dystrophies, as well as markers across 75 other genes associated with keratoconus (KC) and related diseases.
Now that we know the technology exists, we need to recognise the test’s utility and how we can use it in our practices.
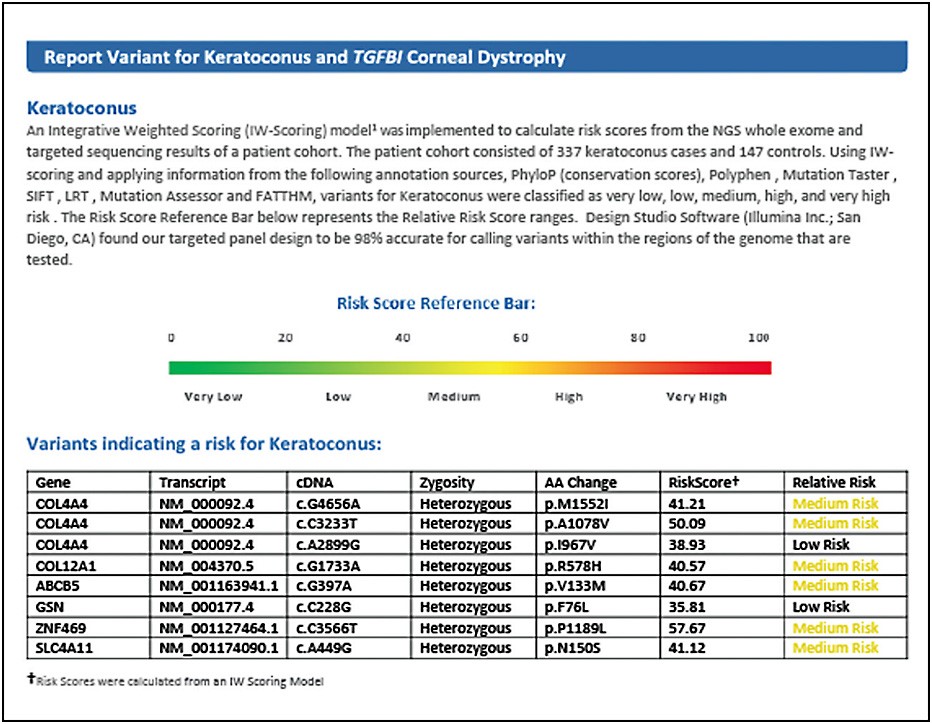
The result reveals that this patient has multiple genes that have been present in KC patients. Of particular concern are the “COL” or “collagen-related” genes, with which polymorphism of these specific genes have been seen in up to 4% of KC patients.
Who are the candidates for corneal genetic testing?
The first candidates to start with are those we have already identified as having the disease. This approach may seem counterintuitive, but since we know they have the disease, we can positively recognise the genetic markers associated with their progression. Keratoconus (KC) has a strong link to positive family history (i.e., genetic link). By finding the markers that have affected the diagnosed patient, I can now test the family members who may be at risk, especially offsprings and siblings. The fastest progression for KC have a tendency to to be between the ages of 13 and 30, and the earlier that these patients can be known and monitored, the more choices they have for intervention as corneal crosslinking if the disease shows signs of progressing.
The International Keratoconus Academy (IKA) is doing excellent effort using imaging diagnostics to screen youths in the Chicago area and finding higher rates of KC than initially anticipated. These findings are based on currently available imaging and corneal curvature measurements. Their testing further demonstrates the theory that the more we test patients, disease detection occurs earlier and more frequently. In the cases of KC, the earlier the detection, further choices for monitoring, prevention, and potential treatments.
The next patient group is those with high mean corneal curvatures and astigmatism rates that exhibit changes over time, especially concerning in younger patients. There are over 300 million individuals globally that could be considered at the pre-clinical stage for KC. Remarkably, more reports are demonstrating that patients can progress well into their 50s and even later. Understanding the patient’s genetic predisposition to the continued progression of KC could certainly add a dimension to the clinical decision-making process.
The final patient groups might be those considering corneal refractive surgery with laser vision correction (LVC). As clinicians, we often encounter “borderline” candidates, and their genetic corneal profile could be a useful supplementary tool to help determine eligibility for LVC.
A Treasured Tool
In conclusion, genetic testing is now showing to be an essential player for anterior segment specialists. In particular, KC genetic screening has very exceptional consequences, from preventing needless surgically induced corneal ectasia to recognising high-risk patients (i.e., family members of KC patients) sooner and screening patients who have high astigmatism earlier and halting their disease with collagen crosslinking sooner. The capability to find gene variants that are related to KC is powerful for clinicians, especially as it relates to potential treatments and follow-ups.
References
- Grady D. Gene Discovery May Lead to Test for Devastating Eye Disorder. New York Times. March 4, 1997. https://www.nytimes.com/1997/03/04/science/gene-discovery-may-lead-to-test-for-devastating-eye-disorder.html . Last accessed on May 15, 2020.
- Wolkoff L. Researchers track the complex roots of glaucoma in DNA. Ocular Surgery News. 2003; DOI https://www.healio.com/ophthalmology/glaucoma/news/print/ocular-surgery-news/%7Bc6acd348-1e62-406d-bfa0-a41c91b8e158%7D/researchers-track-the-complex-roots-of-glaucoma-in-dna .